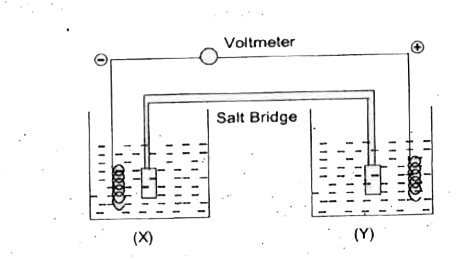
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The cell potential for the unbalanced chemical reaction: Hg(2)^(2+)+...
Text Solution
|
- The standard reduction potential of the reaction H(2)O+e^(-) rarr 1/2 ...
Text Solution
|
- In the reaction HNO(3)+H(2)O hArr H(2)O ^(+) +NO(3)^(-) the conju...
Text Solution
|
- Balance the given Chemical Equation: HNO(3) + Ca (OH)(2) to Ca (NO(3...
Text Solution
|
- For the reaction , H(2(g))+Hg(2)Cl(2) to 2Hg((l))+2HCl((aq)) electroch...
Text Solution
|
- Balance the following redox reaction (i) SnO(2)+c toSn+CO (ii) Fe(3)O(...
Text Solution
|
- Balance the following redox reaction (i) SnO(2)+c toSn+CO (ii) Fe(3)O(...
Text Solution
|
- The cell potential for the unbalanced chemical reaction: Hg(2)^(2+)+NO...
Text Solution
|
- The standard reduction potential at 25^(@)C of the reaction 2H(2)O+2...
Text Solution
|