A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
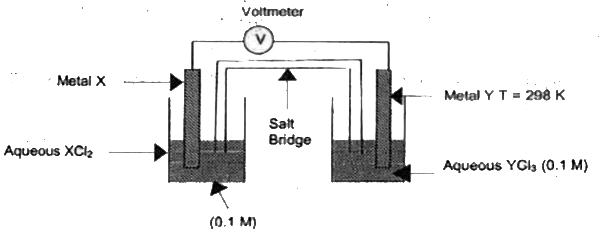
- The following diagram shown as electrochemical cell in which the respe...
Text Solution
|
- The following diagram shows the setup of an electrochemical cell in wh...
Text Solution
|
- 2Cr(s)+3Cu^(2+)(aq)to 2Cr^(3+)(aq)+3Cu(s) दी गई सैल अभिक्रिया को दर्...
Text Solution
|
- एक सेल की सेल अभिक्रिया निम्नलिखित है - Zn(s) +2Ag^+(aq)to Zn^(2+)(a...
Text Solution
|
- निम्नलिखीत सैलों के लिए एनोड अभिक्रिया , कैथोड अभिक्रिया तथा कुल सैल ...
Text Solution
|
- निम्नलिखित अभिक्रिया को सेल आरेख में लिखिए(i) 2Fe(s) + 3Cd^(2+)(aq) ra...
Text Solution
|
- The following diagram shown as electrochemical cell in which the respe...
Text Solution
|
- The following diagram shown as electrochemical cell in which the respe...
Text Solution
|
- The following diagram shown as electrochemical cell in which the respe...
Text Solution
|