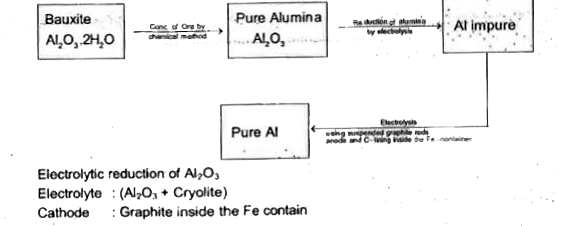
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Extraction of aluminum can be understood by The purpose of ad...
Text Solution
|
- In the extraction of aluminium the purpose of addition of cryolite to ...
Text Solution
|
- Following flow diagram represent the extraction of aluminium form baux...
Text Solution
|
- Write the chemical formula of cryolite. What is the purpose of adding ...
Text Solution
|
- एलुमिना से एल्युमिनियम के निष्कर्षण में क्रायोलाइट का उपयोग किया जाता ...
Text Solution
|
- ऐलुमिनियम के विद्युत-अपघटनी निष्कर्षण में क्रायोलाइट का उपयोग होता है-
Text Solution
|
- एल्यूमिनियम के निष्कर्षण में क्रायोलाइट की क्या उपयोगिता है।
Text Solution
|
- Extraction of aluminum can be understood by The function of fluorspar ...
Text Solution
|
- Extraction of aluminum can be understood by Extraction of metal from t...
Text Solution
|