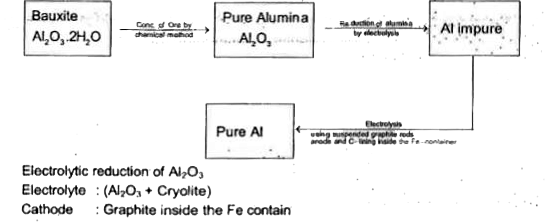
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Extraction of aluminum can be understood by The molten electr...
Text Solution
|
- Extraction of aluminium can be understood by : Electrolytric reduction...
Text Solution
|
- For extraction of sodium from NaCI, the electrolytic mixture NaCI + Na...
Text Solution
|
- Two different electrolytic cells filled with molten Cu(NO(3))(2) a...
Text Solution
|
- One Faraday of electricity is passed through molten Al(2)O(3), aqueous...
Text Solution
|
- Ca, Pb, AI, Na, Mg एवं Cu में से किन-किन धातुओं को विद्युत् अपघटनी विध...
Text Solution
|
- Three faradays of electricity are passed through molten Al2O3 aqueous ...
Text Solution
|
- Extraction of aluminum can be understood by Coke powder is spreaded ov...
Text Solution
|
- অ্যালুমিনিয়াম ধাতুর নিষ্কাশনের সময় গলিত অ্যালুমিনার ওপর কোক-চূর্ণ ছড...
Text Solution
|