A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
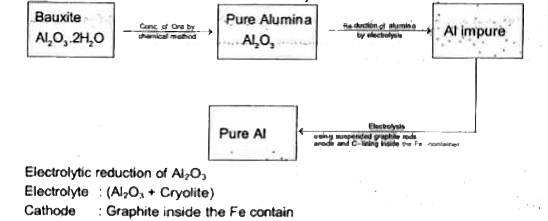
- Extraction of aluminum can be understood by Extraction of met...
Text Solution
|
- Extraction of metal from the ore cassiterite involves
Text Solution
|
- Assertion: Extraction of zinc from sphalerite ore involes the roasting...
Text Solution
|
- Extraction of a metal from ore cassiterite involves :
Text Solution
|
- The metal which can be extracted from pyrolusite ore is:
Text Solution
|
- बौक्साइट अयस्क से एल्युमिनियम के निष्कर्षण की विधि का सचित्र वर्णन कीज...
Text Solution
|
- केसीटेराइट अयस्क से धातु के निष्कर्षण में सम्मिलित है:
Text Solution
|
- ऐल्युमिनियम का निष्कर्षण उसके कार्बोनेट अयस्क से किया जाता है।
Text Solution
|
- Extraction of metal from the ore cassiterite involves
Text Solution
|