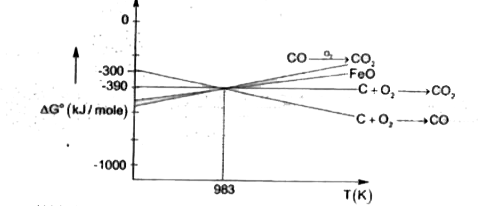
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In Blast Furnace Fe(2)O(3) is reduced to FeO in reduction zone by CO, ...
Text Solution
|
- The temperature of blast furnace to produce iron from its ore Fe(2)O(3...
Text Solution
|
- Metallurgy of Fe is carried in Blast furnace. Blast furnace is divide...
Text Solution
|
- Metallurgy of Fe is carried in Blast furnace. Blast furnace is divided...
Text Solution
|
- Name the four man zones of blast furnace in the extraction of Fe from ...
Text Solution
|
- Before introducing FeO in blast furance, it is converted to Fe(2)O(3) ...
Text Solution
|
- Out of C and CO, which is a better reducing agent for FeO in the lower...
Text Solution
|
- Write the reactions of (1) reduction of Fe(2)O(3) in blast furnace,
Text Solution
|
- Using the Ellingham diagram given below. It is possible to reduce F...
Text Solution
|