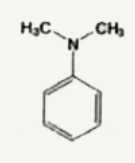
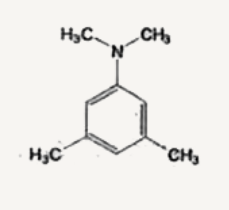
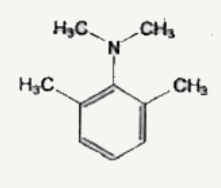
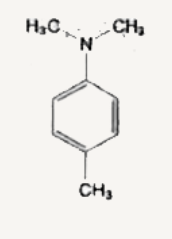
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following has least pk(b), value?
Text Solution
|
- Which of the following has lowest pK(a) value ?
Text Solution
|
- Which of the following acids have the lowest pK(a) value :-
Text Solution
|
- which has the highest pK(a) value ?
Text Solution
|
- The pK(a) " pK(b) values of some bases are as follows. Which is strong...
Text Solution
|
- Which has highest pk(a) value
Text Solution
|
- Which of the following has the highest boiling value pK(a)?
Text Solution
|
- Which has the highest pK(b) value?
Text Solution
|
- Which of the following has the highest pK(b) value?
Text Solution
|