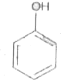
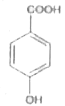
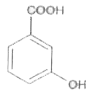
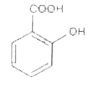
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Acid strength is measured by the position of equilibrium of ionization...
Text Solution
|
- The Bronsted acid which gives the weakest conjugated base is
Text Solution
|
- The halogen acid which produces the weakest conjugate base is
Text Solution
|
- Acid strength of the conjugate acids of the following are
Text Solution
|
- Acid are those compounds which give proton and base are those compound...
Text Solution
|
- Acidic and basic nature of organic compound depends upon-Inductive eff...
Text Solution
|
- Acid strength is measured by the position of equilibrium of ionization...
Text Solution
|
- अभिकथन : दुर्बल अम्लों के संयुग्मी अम्ल बहुत प्रबल संयुग्मी क्षार होते...
Text Solution
|
- Inductive effect influences the basic strength as well as acidic stren...
Text Solution
|