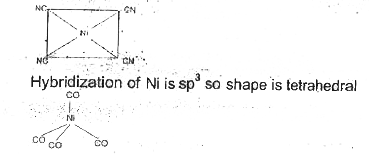Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
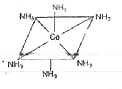
- Draw the structures of [Co(NH(3))(6)]^(3+),[Ni(CN)(4)]^(2-) and [Ni(CO...
Text Solution
|
- Among [Ni(CO)(4)],[Ni(CN)(4)]^(2-) and [NiBr(4)]^(2-) species, the hyb...
Text Solution
|
- Assertion Ni(CO)(4) is tetrahedral in shape Reason Ni atom is in zero ...
Text Solution
|
- Draw the structures of [Co(NH(3))(6)]^(3+),[Ni(CN)(4)]^(2-) and [Ni(CO...
Text Solution
|
- How many of the following are diamagnetic? [Ag(NH(3))(2)]^(+),[Cd(NH(3...
Text Solution
|
- Calculate the effective atomic number of the metal atoms in the follow...
Text Solution
|
- Among [Ni(CN)(4)]^(4-), [Ni(PPh(3))(3)Br] and [Ni(dmg)(2)] species, th...
Text Solution
|
- The hybridisation of Ni in [Ni(CO)(4)] is
Text Solution
|
- [Ni(CO)(4)], [Ni(CN)(4)]^(2-), [NiCl(4)]^(2-) में Ni परमाणु पर संकरण क...
Text Solution
|