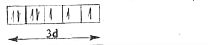Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Assertion[Fe(H(2)O)(5)NO]SO(4) is paramagnetic Reason The Fe in [Fe...
Text Solution
|
- Statement I [Fe(H(2)O)(5)NO]SO(4) is paramagnetic Statement II The Fe ...
Text Solution
|
- Brown coloured ring formed in nitrate test has formula [Fe(H(2)O)(5)NO...
Text Solution
|
- The oxidation state of Fe in brown complex [Fe(H(2)O)(5)NO]SO(4) is
Text Solution
|
- जटिल यौगिक [Fe(H(2)O)(5)NO] SO(4) में Fe के अयुग्मित इलेक्ट्रॉनों की स...
Text Solution
|
- The oxidation state of Fe in brown complex [Fe(H(2)O)(5)NO]SO(4) is
Text Solution
|
- The oxidation state of Fe in brown complex [Fe(H(2)O)(5)NO]SO(4) is
Text Solution
|
- Statement I [Fe(H(2)O)(5)NO]SO(4) is paramagnetic Statement II The F...
Text Solution
|
- Statement-1: [Fe(H(2)O)(5)NO]SO(4) is paramagnetic. Statement-2: The F...
Text Solution
|