Similar Questions
Explore conceptually related problems
Recommended Questions
- The carboncation is unstable whereas carbocation F(3)overset(+)C is m...
Text Solution
|
- Which of the following is the rearranged more stable carbocation of th...
Text Solution
|
- Explain why (CH(3))(3)overset(+)Cis more stable than CH(3)overset(+)CH...
Text Solution
|
- Under common reaction conditions, a carboncation rearranges to another...
Text Solution
|
- Indicate more stable carbocation and explain :
Text Solution
|
- Which of the following is the arranged more stable carbocation of the ...
Text Solution
|
- Explain why (CH(3))(3)overset(+)(C) is more stable than CH(3)overset(+...
Text Solution
|
- The carbocation CH(3)overset(+)(C)HCH(3) is less stable than
Text Solution
|
- Methane is quite stable, whereas silane is unstable? Because -
Text Solution
|
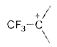 is unstable whereas carbocation `F_(3)overset(+)C` is more stable. Explain
is unstable whereas carbocation `F_(3)overset(+)C` is more stable. Explain