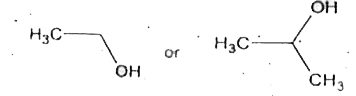Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
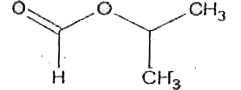
- An ester 'A' (C(4)H(8)O(2)) on treatment with excess methyl magnesium ...
Text Solution
|
- An ester A(C4 H8 O2) , on treatement with excess of methyl magnesium b...
Text Solution
|
- alpha-chloropropanoic acid on treatment with alcoholic acid KOH follow...
Text Solution
|
- Compound (A) C(8)H(6)O(2) on treatment with aq. NaOH followed by acidi...
Text Solution
|
- An ester (A) (C4H8O2) , on treatment with excess methyl magnesium chlo...
Text Solution
|
- An ester 'A' (C(4)H(8)O(2)) on treatment with excess methyl magnesium ...
Text Solution
|
- कोई ऐस्टर (A) C(4)H(8)O(2), मेथिल मैग्नीशियम क्लोराइड की अधिकता के साथ...
Text Solution
|
- An organic compound A (molecular formula C(8)H(16)O(2) ) was hydrolyse...
Text Solution
|
- An organic compound (A) , C(8)H(10)O. The compound (B) upon treatment ...
Text Solution
|