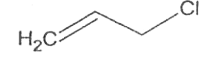
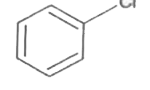
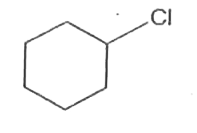
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The organic halide that does not under go any substitution reaction is
Text Solution
|
- In their nucleophilic substitution reactions, aryl halide resembles
Text Solution
|
- Substitution reactions of alkyl halide are initiated by,
Text Solution
|
- Which of the following does not under go HVZ reaction ?
Text Solution
|
- ऐल्किल हैलाइड में नाभिकस्नेही प्रतिस्थापन अभिक्रिया कैसे होती है ? इसक...
Text Solution
|
- Why do haloalkenes under go nucleophillic substitution whereas haloare...
Text Solution
|
- ………………………. alkyl halides undergo substitution by SN^2 reaction wherea...
Text Solution
|
- In halogenation Reaction, Electrophilic substitution reaction of aryl ...
Text Solution
|
- Write the products of nucleophilic substitution reaction of alkyl hali...
Text Solution
|