Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
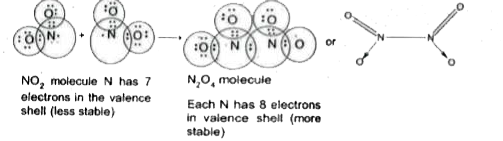
- Why does NO(2) dimerise?
Text Solution
|
- Statement -1 : NO(2) and CIO(2) both being odd electron molecules dime...
Text Solution
|
- 2NO(2) iffN(2)O(4) . The dimerisation of NO(2) is accompanied with :
Text Solution
|
- why does NO(2) dimerise?
Text Solution
|
- Why does NO(2) dimerise ?
Text Solution
|
- Why does NO(2) irritate eyes and nose?
Text Solution
|
- Why does NO(2) dimerise ?
Text Solution
|
- Why does NO(2) dimerise ?
Text Solution
|
- Why does NO(2) dimerise ?
Text Solution
|