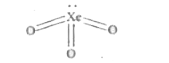
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The noble gases have closed-shell electronic configuration and are mon...
Text Solution
|
- The noble gases have closed-shell electronic cordigaration and are mon...
Text Solution
|
- The noble gases have closed-shell electronic cordigaration and are mon...
Text Solution
|
- The noble gases have closed- shell electronic configuration and are mo...
Text Solution
|
- The noble gases have close shell electronic configuration and are mono...
Text Solution
|
- The noble gases have close shell electronic configuration and are mono...
Text Solution
|
- Statement-1 :Noble gases have very low boiling points. Statement-2 :...
Text Solution
|
- The noble gases have closed-shell electronic configuration and are mon...
Text Solution
|
- The noble gases have closed-shell electronic configuration and are mon...
Text Solution
|