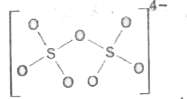
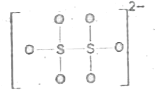
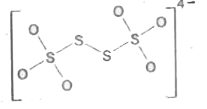
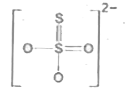
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which is incorrect representation of the dithionate ion structure?
Text Solution
|
- The most unlikely representation of resonance structure of p-nitrophen...
Text Solution
|
- The most unlikely representation of resonance structures of p-nitrophe...
Text Solution
|
- Which of the following is the best representation of the structure of ...
Text Solution
|
- निम्नलिखित में से कौन-सी संरचना कार्बोक्सिलेट आयन का सर्वोत्तम निरूपण ...
Text Solution
|
- The correct structure representation of carboxylate ion is
Text Solution
|
- Which Lewis dot structure is the best representation of the bonding in...
Text Solution
|
- Which is incorrect representation of the dithionate ion structure?
Text Solution
|
- कार्बोक्सिलेट आयन की संरचना का सबसे अच्छा निरूपण है
Text Solution
|