Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
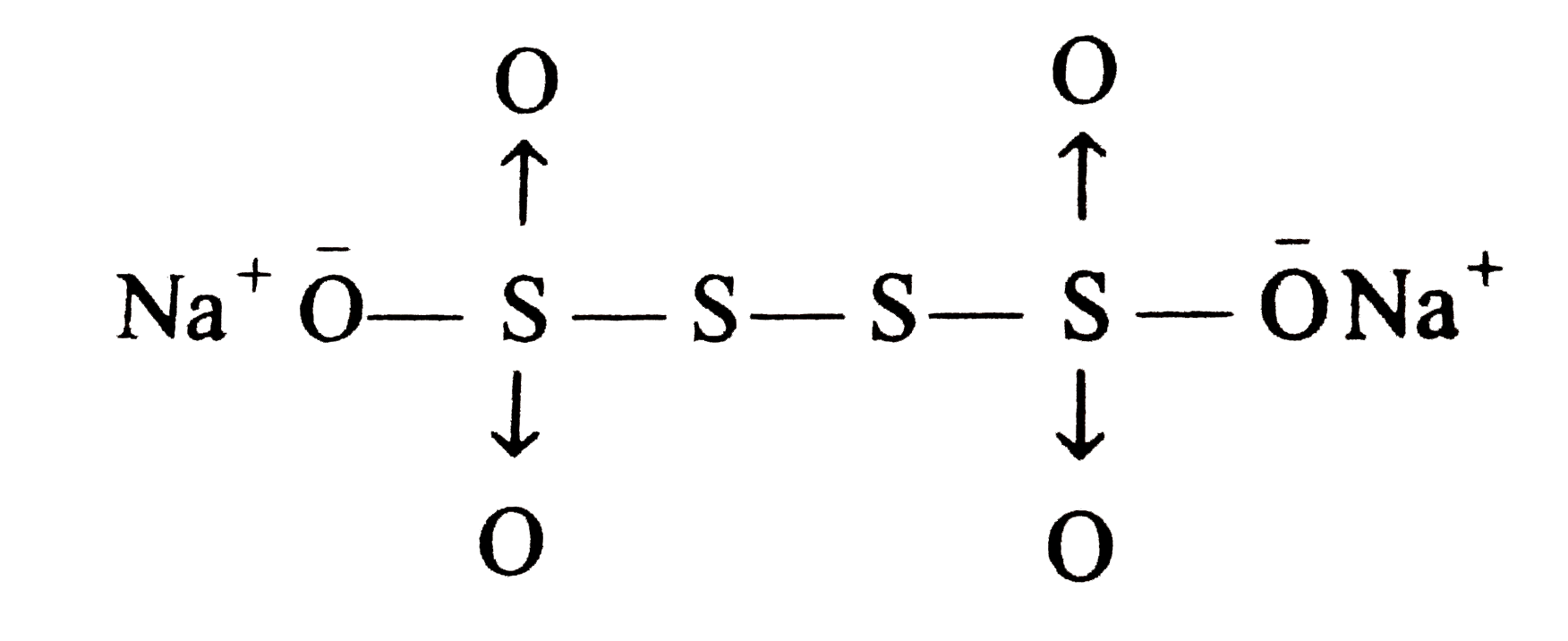
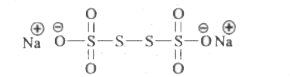
- The difference in the oxidation numbers of the two types of sulphur at...
Text Solution
|
- The difference in the oxidation numbers of two types of sulphul atoms ...
Text Solution
|
- The oxidation number of sulphur in Na(2)S(4)O(6) is
Text Solution
|
- The oxidation number of sulphur in Na(2)S(4)O(6) is
Text Solution
|
- The difference in the oxidation numbers of the two types of sulphur at...
Text Solution
|
- Na(2)S(4)O(6) में दो प्रकार के सल्फर परमाणुओं की ऑक्सीकरण संख्याओं मे...
Text Solution
|
- The average oxidation state of sulphur in Na(2)S(4)O(6) is
Text Solution
|
- The difference in the oxidation number of two types of sulphur atoms i...
Text Solution
|
- The difference in the oxidation number of the two types of suplhur ato...
Text Solution
|