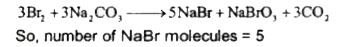Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Reaction of Br(2) with Na(2)CO(3) in aqueous solution gives sodium bro...
Text Solution
|
- Reaction of Br(2) with Na(2)CO(3) in aqueous solution gives NaBr and s...
Text Solution
|
- The answer to each of the following questions is a single digit intege...
Text Solution
|
- Reaction of Br(2) with Na(2)CO(3) in aquesous solution gives sodium br...
Text Solution
|
- Reaction of methyl bromide with aqueous sodium hydroxide involves
Text Solution
|
- Br(2) की NaCO(3) के जलीय विलयन के साथ अभिक्रया से CO(2) गैस निष्कासन क...
Text Solution
|
- Reaction of Br(2) with Na(2)CO(3) in aqueous solution gives sodium br...
Text Solution
|
- Reaction of Br(2) with Na(2)CO(3) in aqueous solution gives sodiu brom...
Text Solution
|
- जलीय विलयन में Br(2) व Na(2)CO(3) की क्रिया सोडियम ब्रोमाइड व सोडियम ब...
Text Solution
|