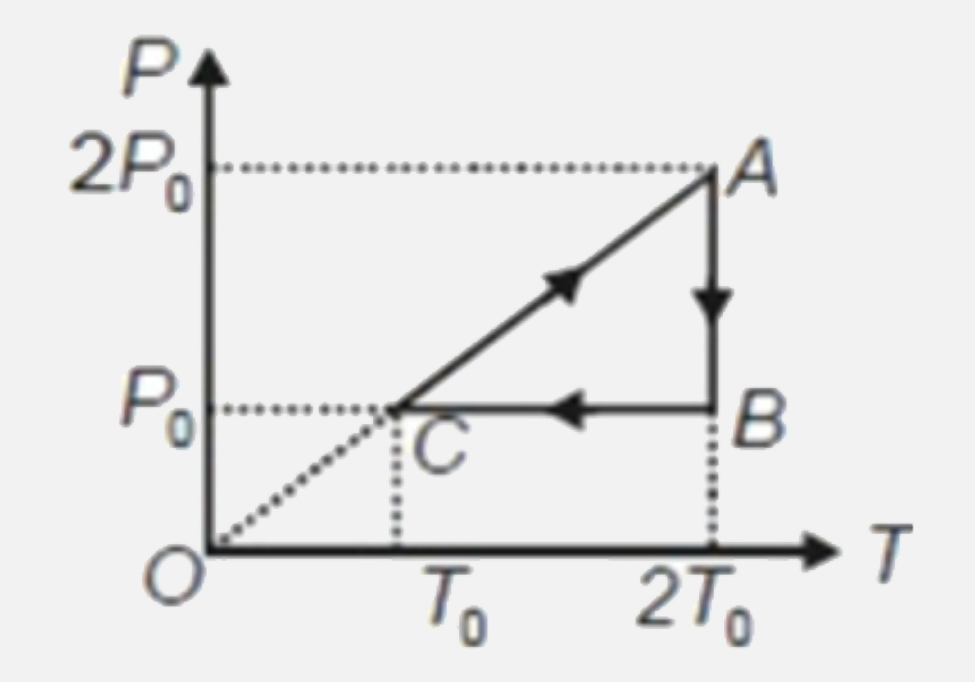
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Three moles of an ideal gas undergo a cyclic process shown in figure. ...
Text Solution
|
- Two moles of a monoatomic ideal gas undergoes a process AB as shown in...
Text Solution
|
- Two moles of Helium gas undergo a reversible cyclic process as shown i...
Text Solution
|
- The value of |[a,a+d,a+2d] , [a+d,a+2d,a+3d] , [a+2d,a+3d,a+4d]|+|[b,b...
Text Solution
|
- The correct order of size among Br^(+), Br, Br^(-)
Text Solution
|
- 1) Fragmentation () a) Planaria br 2) Unripe fruits () b) Yeast br 3) ...
Text Solution
|
- వేర్ల ద్వారా శాఖీయోత్పత్తి జరిపే మొక్కbr i) డాలియాbr ii) ముల్లంగ...
Text Solution
|
- An ideal gas is taken in a cyclic process as shown in the figure. Calc...
Text Solution
|
- Two moles of helium gas undergo a cyclic process as shown in figure. A...
Text Solution
|