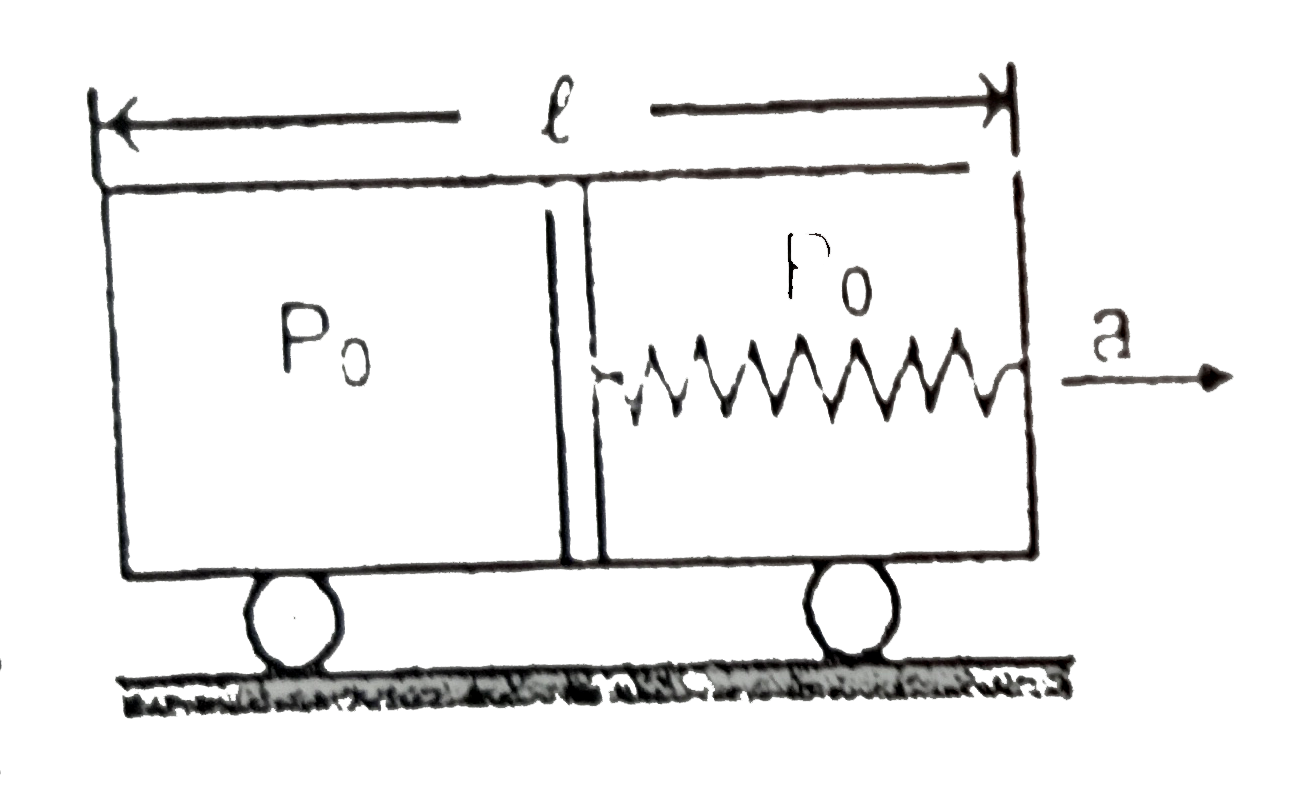
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An adiabatic piston of mass m equally divides an insulated container o...
Text Solution
|
- A fixed container is fitted with a piston which is attached to a sprin...
Text Solution
|
- A closed and isolated cylinder contains ideal gas. An adiabatic separa...
Text Solution
|
- A container has a tight fitting movable piston which can slide without...
Text Solution
|
- A cylinder contains equal volumes of Ar and H(2), separated by a freel...
Text Solution
|
- An adiabatic cylinder has length 2 L and cross sectional area A. A fre...
Text Solution
|
- An adiabatic piston of mass m equally divides an insulated container o...
Text Solution
|
- Consider the adjacent figure. A piston divides a cylindrical container...
Text Solution
|
- An ideal gas is enclosed in a thermally insulated container. Container...
Text Solution
|