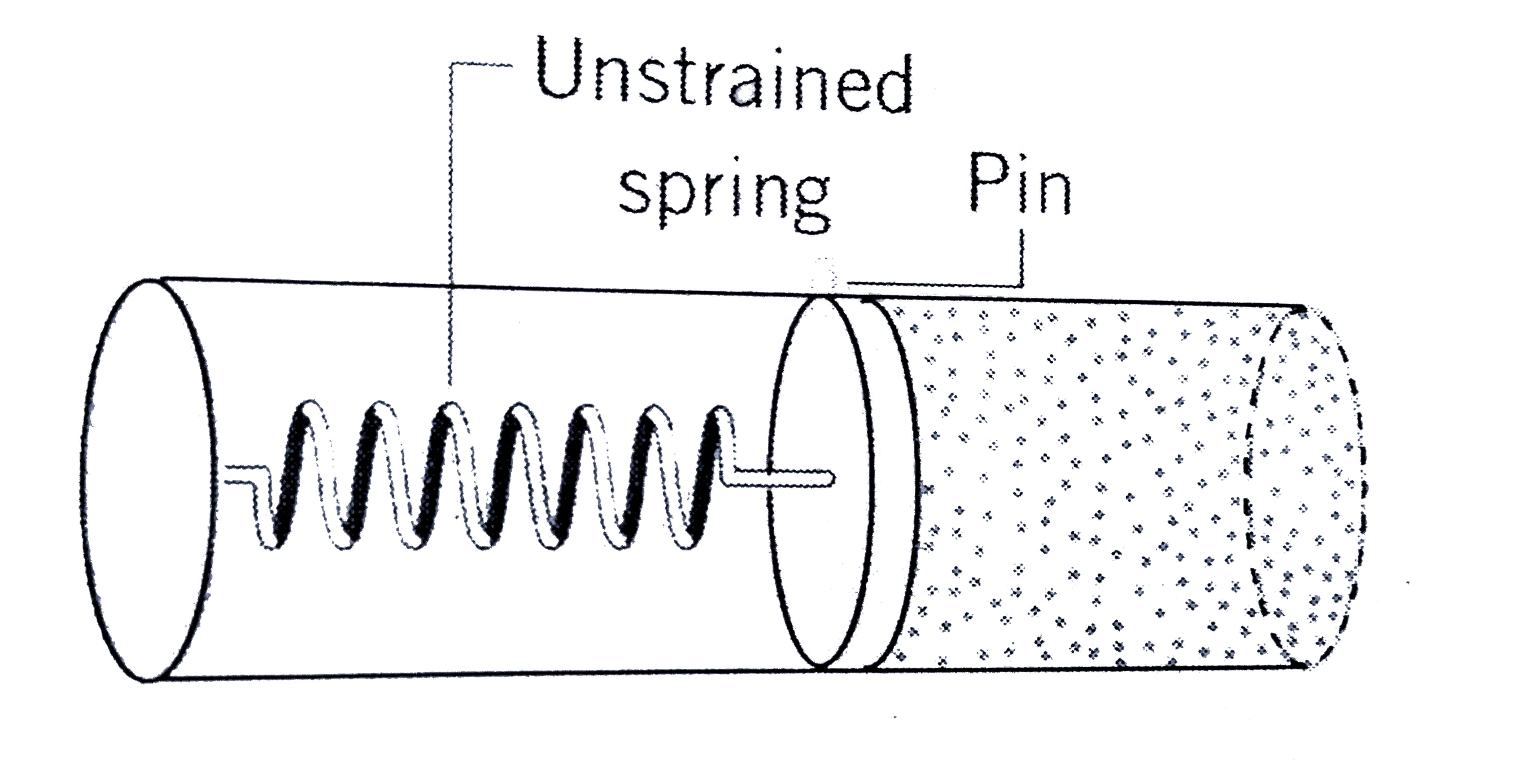
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A gas fills the right portion of a horizontal cylinder whose radius is...
Text Solution
|
- An ideal gas is enclosed in a cylinder with a movable piston on top of...
Text Solution
|
- A gas is filled in the cylinder shown in fig. The two pistons are join...
Text Solution
|
- Comprehension-2 A mono atomic ideal gas is filled in a non conducting...
Text Solution
|
- A gas fills the right portion of a horizontal cylinder whose radius is...
Text Solution
|
- A gas filled in a cylinder fitted with movable piston is allowed to ex...
Text Solution
|
- A movable piston separates a sample of gas from vacuum. In this state,...
Text Solution
|
- A gas is filled in a cylinder fitted with a piston at a definite tempe...
Text Solution
|
- एक-परमाणुक आदर्श गैस प्रारम्भिक ताप T(3) पर, एक पिस्टन युक्त सिलिण्डर ...
Text Solution
|