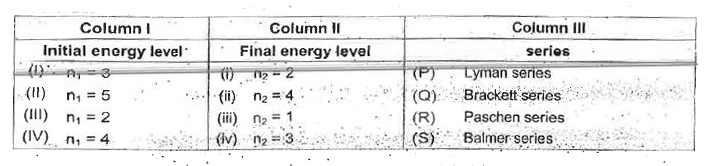
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In Bohr Atomic model regarding transition of electron for hydrogen lik...
Text Solution
|
- In Bohr model of the hydrogen atom, the lowest orbit corresponds to
Text Solution
|
- What is the change in the orbit radius when the electron in the hydrog...
Text Solution
|
- Which electronic transition in a hydrogen atom releases the greatest a...
Text Solution
|
- Which of the following electron transitions in a hydrogen atom will re...
Text Solution
|
- Which of the following transitions of an electron in hydrogen atom emi...
Text Solution
|
- Which of the following transitions of electrons in the hydrogen atom w...
Text Solution
|
- Which of the following transitions of an electron in hydrogen atom emi...
Text Solution
|
- in the Bohr hydrogen atom, the electronic transition emmiting light of...
Text Solution
|