Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
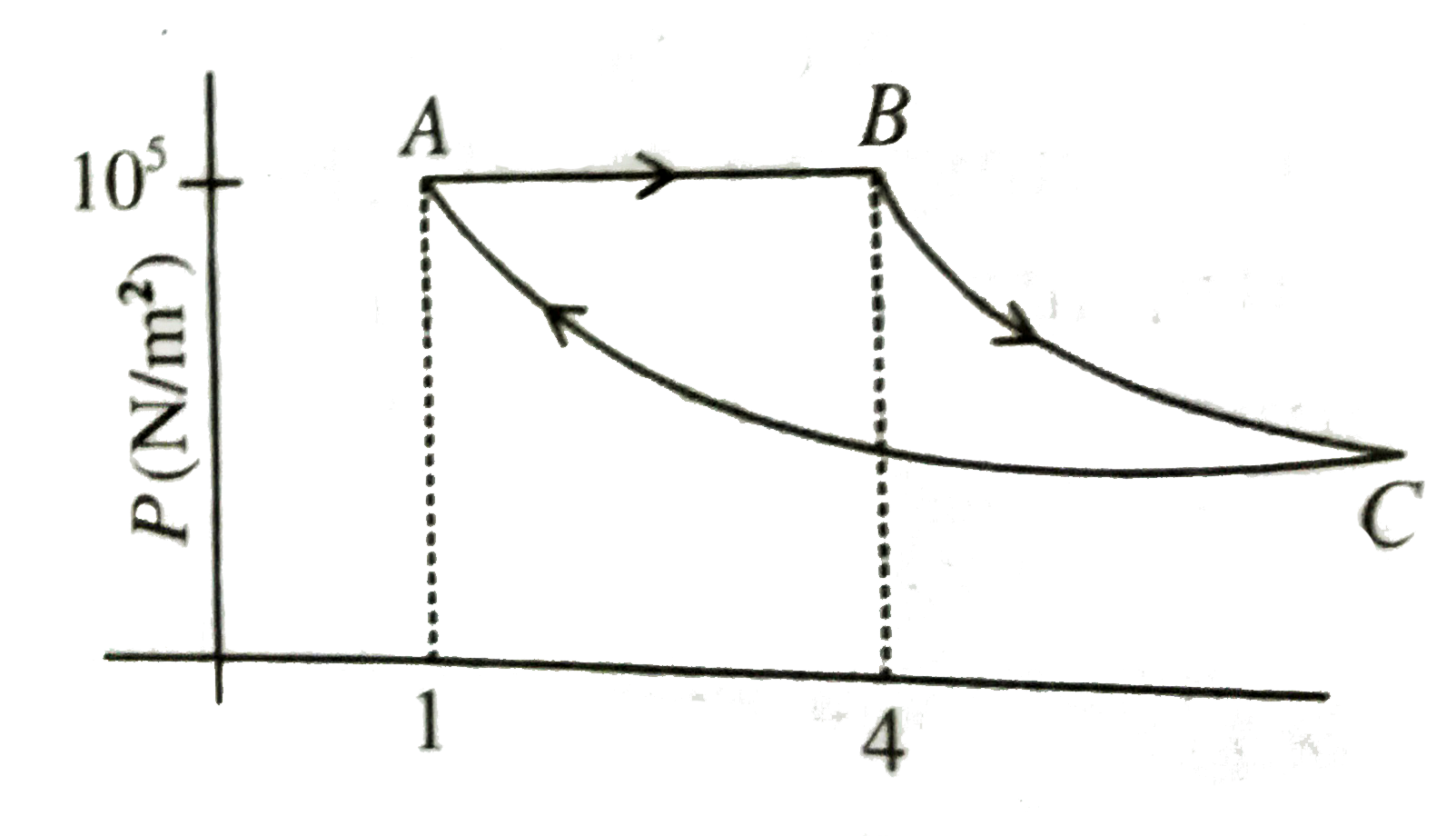
- A fixed mass of gas is taken through a process A rarr B rarr C rarrA. ...
Text Solution
|
- An ideal gas (2.0 moles) is carried round a cycle as shown. If the pro...
Text Solution
|
- A fixed mass of gas is taken through a process A rarr B rarr C rarrA. ...
Text Solution
|
- A fixed mass of gas is taken through a process A rarr B rarr C rarrA ....
Text Solution
|
- A fixed mass of gas is taken through a process A rarr B rarr C rarrA ....
Text Solution
|
- An ideal gas undergoes a series of processes represented bya rarr B ra...
Text Solution
|
- Isobaric expansion from A rarr B , isochoric pressure drop from B rarr...
Text Solution
|
- Five moles of gas is put through a series of changes as shown graphica...
Text Solution
|
- An ideal gas is taken through the cycle A rarr B rarr C rarr A , as sh...
Text Solution
|