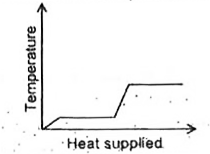
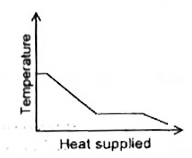
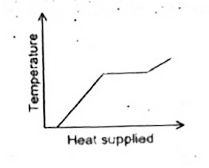
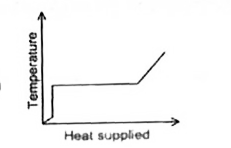
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- A block of ice at -8^(@)C is slowly heated and converted to steam ...
Text Solution
|
- A block of ice at -10^@C is slowly heated and converted to steam at 10...
Text Solution
|
- A block of ice at -12^(@)C is slowly heated and converted into steam a...
Text Solution
|
- -10^(@)C पर स्थित बर्फ के एक टुकड़े को धीरे धीरे गर्म करके 100^(@)C ताप...
Text Solution
|
- -20^(@)C तापमान का बर्फ के एक ब्लॉक (block) को धीरे-धीरे गर्म किया जात...
Text Solution
|
- A block of ice at -5^@C is slowly heated and converted to steam at 10...
Text Solution
|
- A block of ice -10^(@)C is slowly heated and converted to steam at 100...
Text Solution
|
- A block of ice at temperature -20^@ C is slowly heated and converted t...
Text Solution
|
- A block at - 10^@C is slowly heated and converted to steam at 100^(@)C...
Text Solution
|