Similar Questions
Explore conceptually related problems
Recommended Questions
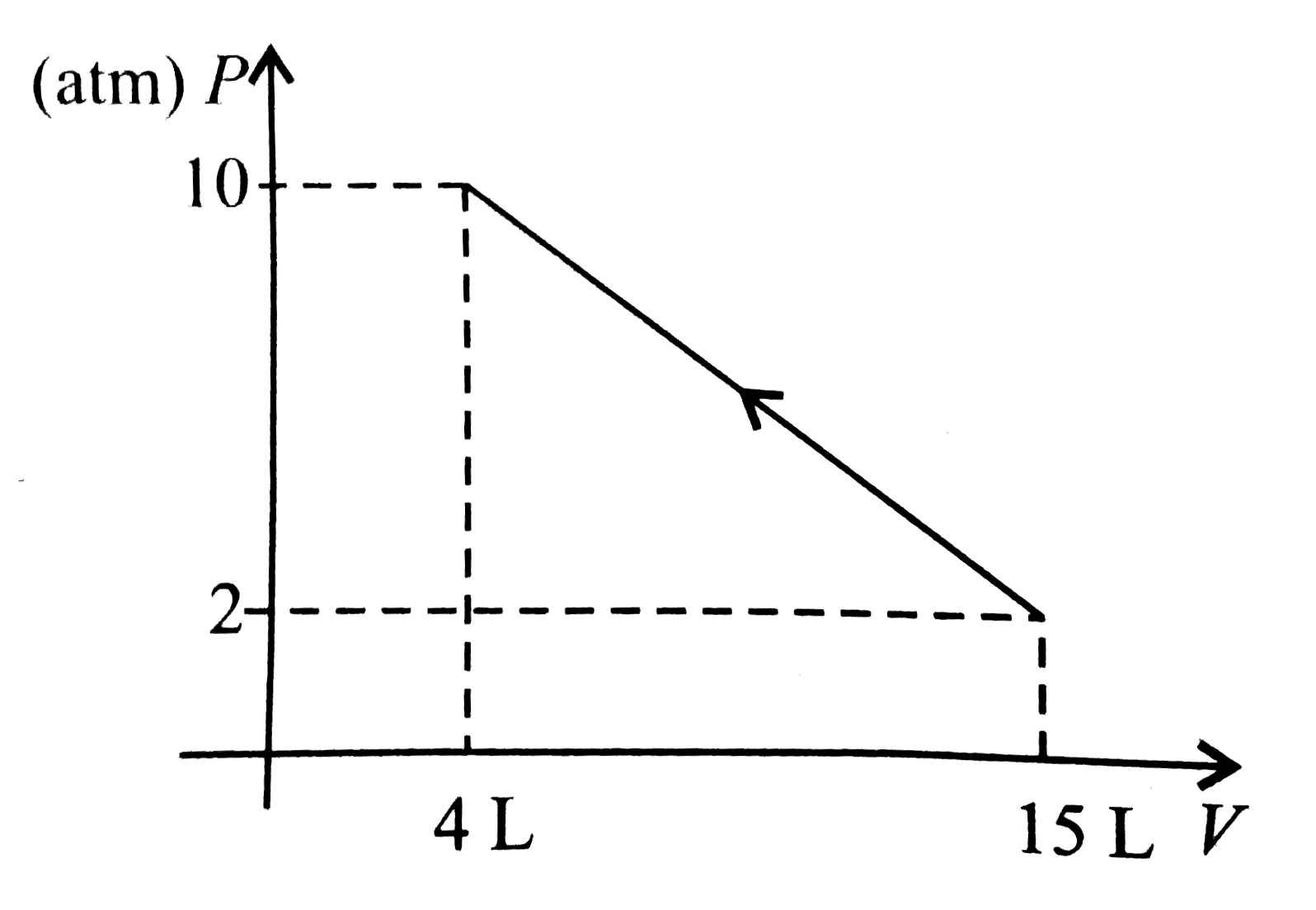
- 1 mol of a gas is changed from its initial state (15 L, 2 atm) to fina...
Text Solution
|
- 1 mol of a gas is changed from its initial state (15 L, 2 atm) to fina...
Text Solution
|
- Two moles of an ideal gas undergo the following process : (a) a reve...
Text Solution
|
- Calculate DeltaU for a gas, if enthalpy change is 40 atm-L for the sta...
Text Solution
|
- Calculate DeltaH (in atm-litre) for a real gas undergoing a change fr...
Text Solution
|
- One mole of an ideal gas undergoes a change of state (2.0) atm, 3.0 L)...
Text Solution
|
- One mole of a gas changed from its initial state (15L,2 atm) to final ...
Text Solution
|
- When a sample of ideal gas is changed from an initial state to a final...
Text Solution
|
- A system containing real gas changes it's state from state -1 to state...
Text Solution
|