A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
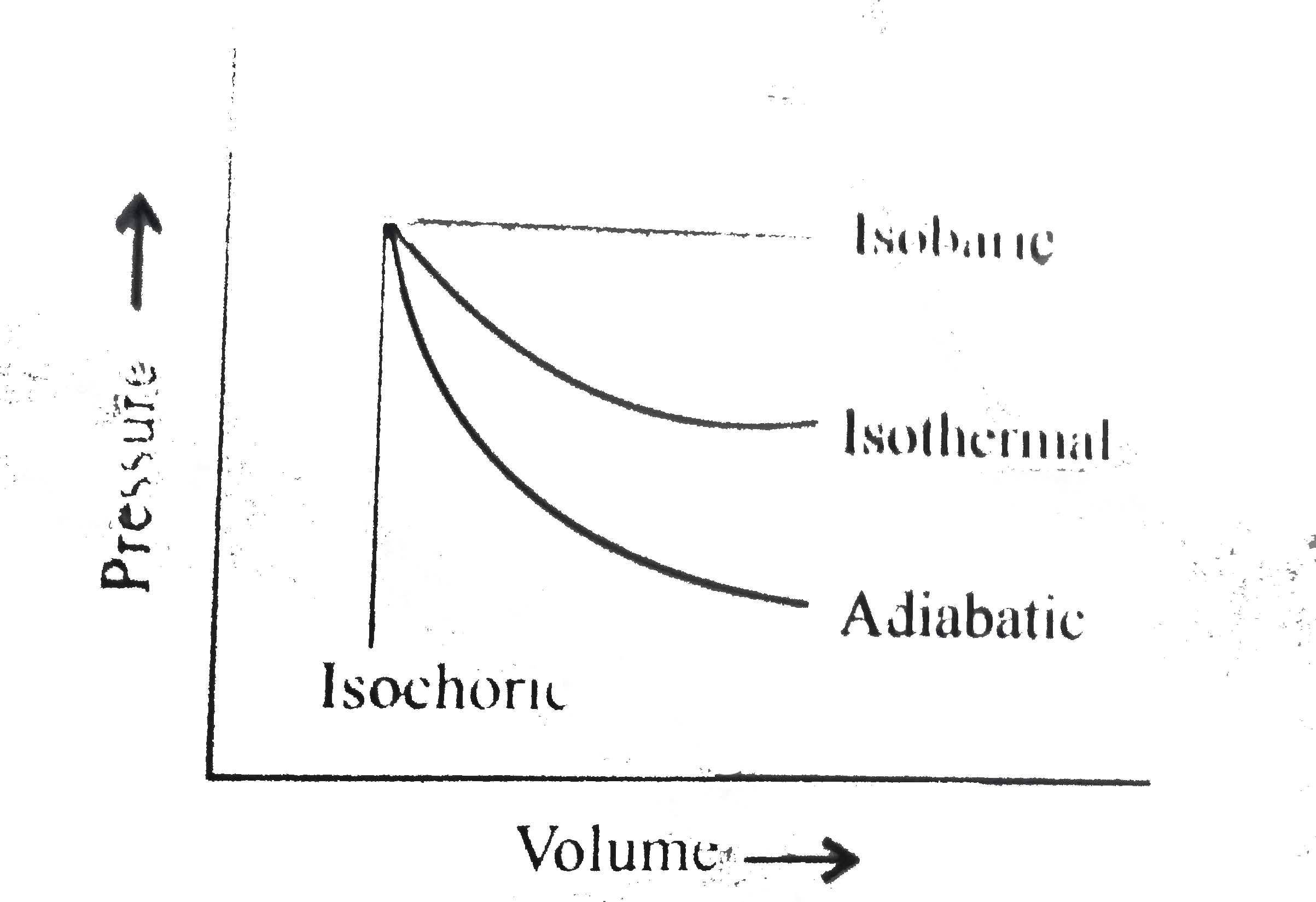
- The pressure-volume of varies thermodynamic process is shown in graphs...
Text Solution
|
- A sample of ideal gas undergoes isothermal expansion in a reversible m...
Text Solution
|
- The pressure-volume of varies thermodynamic process is shown in graphs...
Text Solution
|
- The pressure-volume of varies thermodynamic process is shown in graphs...
Text Solution
|
- The pressure-volume of varies thermodynamic process is shown in graphs...
Text Solution
|
- The pressure-volume of varies thermodynamic process is shown in graphs...
Text Solution
|
- The pressure-volume of varies thermodynamic process is shown in graphs...
Text Solution
|
- If w(1).w(2),w(3) and w(4) are work done in isothermal, adiabatic, iso...
Text Solution
|
- Work is the mode of transference of energy. If the system involves gas...
Text Solution
|