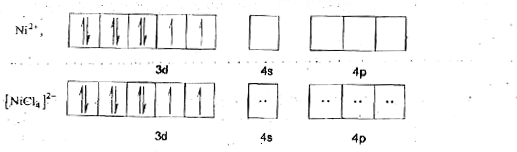
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The compound having a tetrahedral geometry is .
Text Solution
|
- The compound having a tetrahedral geometry is .
Text Solution
|
- The compound having tetrahedral geometry is
Text Solution
|
- Which of the following does not have regular tetrahedral geometry ?
Text Solution
|
- किस आयन की ज्यामिति चतुष्फलकीय है :
Text Solution
|
- निम्नलिखित में से किसकी चतुष्फलकीय ज्यामिति है -
Text Solution
|
- Tetrahedral geometry of a coordination compound involves the following...
Text Solution
|
- Which of the following compound has tetrahedral geometry?
Text Solution
|
- Which of the following complex does not have tetrahedral geometry ?
Text Solution
|