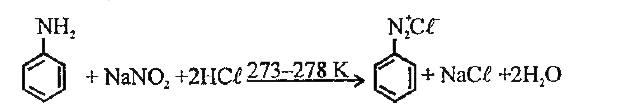Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-THE SOLID STATE-MULTIPLE CHOICE QUESTIONS
- How will you distinguish between the following pairs of term ? Tetra...
Text Solution
|
- The major binding force of diamond , silicon and quartz is
Text Solution
|
- Graphite is an example of:
Text Solution
|
- The total number of lattice arrangements in different crystal system i...
Text Solution
|
- Which one of the following will have a low heat of fusion ?
Text Solution
|
- The crystal system of a compound with unit cell parameter,
Text Solution
|
- Zn converts its melting state to its solid state, it has hcp structure...
Text Solution
|
- A metallic crystal crystallises into a lattice containing sequence ofl...
Text Solution
|
- The cordination number of a metal crystallising in a hexagonal close-p...
Text Solution
|
- The packing fraction for a body centred cube is
Text Solution
|
- In crystal structure of sodium chloride, the arrangement of Cl ions is
Text Solution
|
- The percentage of nitrogen in HNO3 is ...................................
Text Solution
|
- The number of octahedral sites per sphere in fcc structure is
Text Solution
|
- The number of tetrahedral sites per sphere in fcc structure is
Text Solution
|
- A crystal lattice with alternate +ve and-ve ions has radius ratio of 0...
Text Solution
|
- Calculate the equivalent weight of oxalic acid
Text Solution
|
- Which of the following is Bragg's equation?
Text Solution
|
- 74.5g of metal chloride contains 35.5g of chlorine. the equivalent wei...
Text Solution
|
- Name the element with electronic configuration 1s2 2s2
Text Solution
|
- The units of molality are ...........
Text Solution
|
- How many gram of oxygen is required to completely react with 0.200g of...
Text Solution
|