Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-ALCOHOLS, PHENOLS AND ETHERS-MULTIPLE CHOICE QUESTION
- Phenyl methyl ether reacts with HI to give phenol and methyl iodide an...
Text Solution
|
- In B(2)H(6) , B-atom is
Text Solution
|
- What is fermentation?
Text Solution
|
- What happens when phenol is treated with benzoyl chloride in the prese...
Text Solution
|
- Rust is mixture of:
Text Solution
|
- In glycerine,
Text Solution
|
- Victor Meyer's test is not given by
Text Solution
|
- When phenol is treated with CHCl(3) and NaOH, the product formed is
Text Solution
|
- C(6)H(5)OHunderset(H(2)SO(4))overset(conc.HNO(3))rarrX The X can be
Text Solution
|
- Which compound is predominantly formed when phenol is allowed to react...
Text Solution
|
- Phenol upon distiliation with zinc dust gives :
Text Solution
|
- Commercial alcohol is made unfit for drinking by adding
Text Solution
|
- The test used to distinguish alcohols from one another is known as
Text Solution
|
- Reaction used for the preparation of ethers is
Text Solution
|
- Which of the following is used to distinguish between 1^(@), 2^(@) and...
Text Solution
|
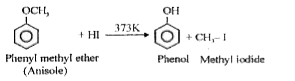 When phenyl methyl ether treated with HI then products are phenol and methyl iodide. As protonation of anisole gives methyl phenyl oxonium ion `C_(6)H_(5)-underset(H)underset(|)overset(+)O-CH_(3)` In this ion bond between `O-CH_(3)` is weaker than the bond between `O - C_(6)H_(5)` (partial double bond character). So attack by `l^(-)` ion breaks weaker `O-CH_(3)` bond forming `CH_(3)-I` and phenol. Phenol thus formed not reacts further to form aryl halides as `sp^(2)` hybridized carbon-atom of phenol do not undergo nucleophilic substitution reaction to form corresponding aryl halides.
When phenyl methyl ether treated with HI then products are phenol and methyl iodide. As protonation of anisole gives methyl phenyl oxonium ion `C_(6)H_(5)-underset(H)underset(|)overset(+)O-CH_(3)` In this ion bond between `O-CH_(3)` is weaker than the bond between `O - C_(6)H_(5)` (partial double bond character). So attack by `l^(-)` ion breaks weaker `O-CH_(3)` bond forming `CH_(3)-I` and phenol. Phenol thus formed not reacts further to form aryl halides as `sp^(2)` hybridized carbon-atom of phenol do not undergo nucleophilic substitution reaction to form corresponding aryl halides.