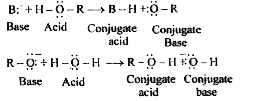Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-ALCOHOLS, PHENOLS AND ETHERS-MULTIPLE CHOICE QUESTION
- Why alcohols are weaker acids than water ?
Text Solution
|
- In B(2)H(6) , B-atom is
Text Solution
|
- What is fermentation?
Text Solution
|
- What happens when phenol is treated with benzoyl chloride in the prese...
Text Solution
|
- Rust is mixture of:
Text Solution
|
- In glycerine,
Text Solution
|
- Victor Meyer's test is not given by
Text Solution
|
- When phenol is treated with CHCl(3) and NaOH, the product formed is
Text Solution
|
- C(6)H(5)OHunderset(H(2)SO(4))overset(conc.HNO(3))rarrX The X can be
Text Solution
|
- Which compound is predominantly formed when phenol is allowed to react...
Text Solution
|
- Phenol upon distiliation with zinc dust gives :
Text Solution
|
- Commercial alcohol is made unfit for drinking by adding
Text Solution
|
- The test used to distinguish alcohols from one another is known as
Text Solution
|
- Reaction used for the preparation of ethers is
Text Solution
|
- Which of the following is used to distinguish between 1^(@), 2^(@) and...
Text Solution
|