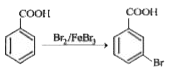Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS -MULTIPLE CHOICE QUESTIONS
- What are macromolecular colloids?
Text Solution
|
- In the following reaction, product P is R - overset(O)overset(||)C -...
Text Solution
|
- Rosenmund's reduction of an acyl chloride gives
Text Solution
|
- The wavelength of a beam of light is 25 micrometer. What is its wavenu...
Text Solution
|
- Which of the following compounds gives a ketone with Grignard's reagen...
Text Solution
|
- Identify the product Y in the sequence. CH3 CHO + CH3 MgBr overset(...
Text Solution
|
- Which of the following reacts with NaOH to produce an acid and an alco...
Text Solution
|
- Which of the following gives aldol condensation reaction?
Text Solution
|
- CH3 - overset(O)overset(||)C - CH2 - CH3, overset(SeO2)to X + H2O here...
Text Solution
|
- The following reaction is known as
Text Solution
|
- What happens when ethanoyl chloride is reduced with H2 in the presence...
Text Solution
|
- If formaldehyde and KOH are heated, then we get
Text Solution
|
- The addition of HCN to carbony compounds ts an example of
Text Solution
|
- Dimerisation in carboxylic acid is due to
Text Solution
|
- Lower carboxylic acids are soluble in water due to
Text Solution
|
- Carboxylic acids are more acidic than phenols and alcohols because of
Text Solution
|
- CH3 COCl can be obtained directly by reacting PCl5 with
Text Solution
|
- HCOOH reacts with conc. H2SO4 to produce
Text Solution
|
- The reaction RCH2 CH2 COOH underset(Br2) overset("Red P")to R - CH2-...
Text Solution
|
- C6H6+CO + HCl overset("Anhy"AlCl3) to X + HCl Compound X is
Text Solution
|
- The chemical reaction of acetaldehyde and ammonia gives
Text Solution
|