Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS -MULTIPLE CHOICE QUESTIONS
- Explain, why acid amides are amphoteric in nature ?
Text Solution
|
- In the following reaction, product P is R - overset(O)overset(||)C -...
Text Solution
|
- Rosenmund's reduction of an acyl chloride gives
Text Solution
|
- The wavelength of a beam of light is 25 micrometer. What is its wavenu...
Text Solution
|
- Which of the following compounds gives a ketone with Grignard's reagen...
Text Solution
|
- Identify the product Y in the sequence. CH3 CHO + CH3 MgBr overset(...
Text Solution
|
- Which of the following reacts with NaOH to produce an acid and an alco...
Text Solution
|
- Which of the following gives aldol condensation reaction?
Text Solution
|
- CH3 - overset(O)overset(||)C - CH2 - CH3, overset(SeO2)to X + H2O here...
Text Solution
|
- The following reaction is known as
Text Solution
|
- What happens when ethanoyl chloride is reduced with H2 in the presence...
Text Solution
|
- If formaldehyde and KOH are heated, then we get
Text Solution
|
- The addition of HCN to carbony compounds ts an example of
Text Solution
|
- Dimerisation in carboxylic acid is due to
Text Solution
|
- Lower carboxylic acids are soluble in water due to
Text Solution
|
- Carboxylic acids are more acidic than phenols and alcohols because of
Text Solution
|
- CH3 COCl can be obtained directly by reacting PCl5 with
Text Solution
|
- HCOOH reacts with conc. H2SO4 to produce
Text Solution
|
- The reaction RCH2 CH2 COOH underset(Br2) overset("Red P")to R - CH2-...
Text Solution
|
- C6H6+CO + HCl overset("Anhy"AlCl3) to X + HCl Compound X is
Text Solution
|
- The chemical reaction of acetaldehyde and ammonia gives
Text Solution
|

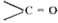 group reduces its basic nature and behaving like acids. Now it can easily lose `H^+` ion due to assignment of positive charge on nitrogen atom. Hence, acid amides are amphoteric in nature.
group reduces its basic nature and behaving like acids. Now it can easily lose `H^+` ion due to assignment of positive charge on nitrogen atom. Hence, acid amides are amphoteric in nature.