Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-SOME BASIC CONCEPTS OF CHEMISTRY-Multiple Choice Questions (MCQs)
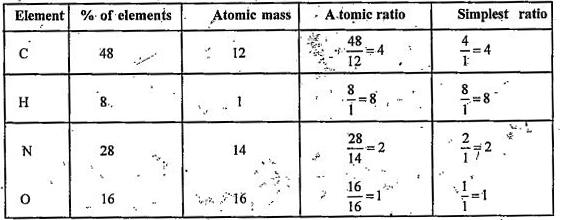
- An organic compound has the following percentage composition : C= 48%,...
Text Solution
|
- How many significant figures are there in each of the following? 6.0...
Text Solution
|
- The prefix 10^(18) is
Text Solution
|
- Fill in the blanks: One fermi =
Text Solution
|
- Which one of the following is not an element?
Text Solution
|
- Law of mulitple proportions is illustrated by one of the following pai...
Text Solution
|
- 1 amu is equal to
Text Solution
|
- Define atomic mass of an element.
Text Solution
|
- How is Avogadro's number denoted ?
Text Solution
|
- Calculate the equivalent weight of oxalic acid
Text Solution
|
- Avogadro's hypothesis is related with
Text Solution
|
- Atomicity of oxygen is
Text Solution
|
- Gram molecular mass is expressed in
Text Solution
|
- 1 mole represents particles.......
Text Solution
|
- Which of the following is incorrect?
Text Solution
|
- In case of molecular substances, one mole represents
Text Solution
|
- Gram molecular mass of H(2)SO(4) is
Text Solution
|
- Calculate the mass of 2.5 gram atoms of magnesium.
Text Solution
|
- 1 mole of oxygen molecules is equal to
Text Solution
|
- Calculate the number of atoms in 0.25 mole atoms of carbon
Text Solution
|
- Calculate the volume occupied at NTP by 14g of nitrogen gas .
Text Solution
|