Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-STRUCTURE OF ATOM -MULTIPLE CHOICE QUESTIONS (MCQ s)
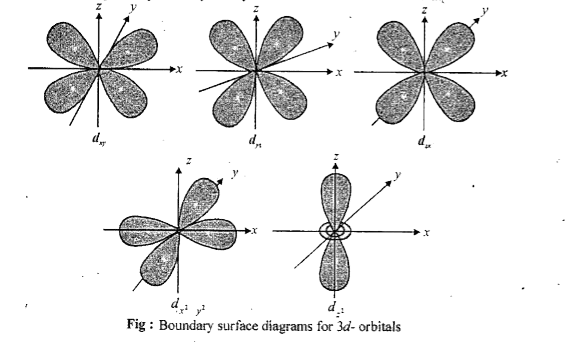
- Draw the shapes of all d-orbitals.
Text Solution
|
- Emission of beta -particle is equivatent to:
Text Solution
|
- The mass of the neutron is of the order of
Text Solution
|
- The number of electrons and neutrons of an element is 18 and 20 respec...
Text Solution
|
- Which of the following has longest wavelength ?
Text Solution
|
- The value of Planck's constant is
Text Solution
|
- Which of the following expresson gives the de-Broglie relationship ?
Text Solution
|
- The total number of orbitals possible for the quantum number n is
Text Solution
|
- The four quantum numbers of the valence electron of potassium are
Text Solution
|
- An electron has principal quantum number 3. The number of its (i) shel...
Text Solution
|
- The correct set of quantum numbers for a 4d electron is
Text Solution
|
- No two electrons in an atom will have all the four quantum numbers sam...
Text Solution
|
- The correct order of increasing energy of atomic orbitals is
Text Solution
|
- The energy of an electron in nth orbit of hydrogen atom is
Text Solution
|
- The triad of nuclei that is isotonic is
Text Solution
|
- Bohr's model can explain
Text Solution
|
- If the value of l = 2, what will be the value of principal quantum num...
Text Solution
|
- Rutherford's experiment on scattering of alpha particles showed for th...
Text Solution
|
- 3d-orbitals have
Text Solution
|
- Number of unpaired electrons in the electronic configuration 1s ^(2) ,...
Text Solution
|
- The two electrons in an orbital have different
Text Solution
|