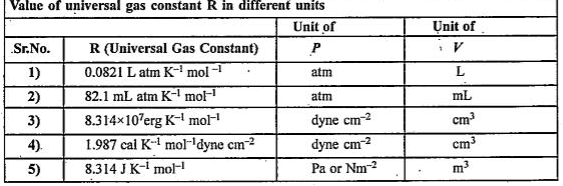
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-STATES OF MATTER -Multiple Choice Questions (MCQs)
- Derive ideal gas equation.
Text Solution
|
- Gas equation PV = nRT is obeyed by
Text Solution
|
- The root mean square velocity of an ideal gas at constant pressure var...
Text Solution
|
- A gas will approach ideal behaviour at
Text Solution
|
- (True/False) The compressibility factor (z) for ideal gases is 1,.
Text Solution
|
- van der Waal equation is true for :
Text Solution
|
- The van der Waal's equation of state reduces itself to the ideal gas e...
Text Solution
|
- When the temperature is raised, the viscosity of the liquid decreases....
Text Solution
|
- The relationship between coefficient of viscosity of a liquid and temp...
Text Solution
|
- Which of the following statement(s) is/are correct, if intermolecular ...
Text Solution
|
- The internal energy of one mole of ideal gas is
Text Solution
|
- An ideal gas obeying kinetic theory of gases can be liquefied if
Text Solution
|
- The number of moles of a substance are given by
Text Solution
|
- According to Boyle's law
Text Solution
|
- The relation, volume of gas (V) prop no. of moles (n), is
Text Solution
|
- Which one is correct?
Text Solution
|
- The unit of R does not depend on
Text Solution
|
- Dalton's law of partial pressure is related-with
Text Solution
|
- The spherical shape of liquid drops is due to
Text Solution
|
- According to Charle's law (here K is constant of proportionality)
Text Solution
|
- When the universal gas constant (R ) is divided by Avogadro's number (...
Text Solution
|