Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-EQUILIBRIUM -Multiple Choice Questions (MCQs)
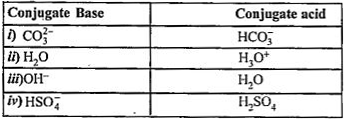
- Write conjugate acids of (i) CO(3)^(2-) (ii) H(2)O (iii) OH^(-) (iv) H...
Text Solution
|
- List the value of n and l for the following orbitals : 4s
Text Solution
|
- For the reaction , 2HI hArr H(2) + I(2)
Text Solution
|
- Equilibrium constant for the reaction 2NO(g) + Cl(2)(g) hArr 2NOCl(g) ...
Text Solution
|
- k(1) and k(2) are the velocity constants of forward and backward react...
Text Solution
|
- The number of electrons present in 3d of Cu+ is ........
Text Solution
|
- Consider the reaction 2CO(g)+O(2)(g)hArr2CO(2)(g)+Heat Under what co...
Text Solution
|
- Using s, p, d notations, describe the orbital with the quantum number....
Text Solution
|
- Using s, p, d notations, describe the orbital with the quantum number....
Text Solution
|
- Which of the following is strongest acid ?
Text Solution
|
- What is the structural formula of 2-Chloro-2-methylpropane?
Text Solution
|
- What is the structural formula of 2-Chlorobutane?
Text Solution
|
- What is the structural formula of 1-Chloro-3-methylpentane?
Text Solution
|
- What is the structural formula for 1-Chloro-2,2-dimethylpropane?
Text Solution
|
- What is the structural formula for Ethylene dibromide?
Text Solution
|
- What is the structural formula of 1-Bromo-2,3-dichlorobutane?
Text Solution
|
- The pH indicators are
Text Solution
|
- What is incorrect about buffer solution ?
Text Solution
|
- Precipitation takes place when the ionic product
Text Solution
|
- Which of the following is an example of heterogeneous equilibrium ?
Text Solution
|
- For the reaction, a A + b B rarr Products. According to law of mass ac...
Text Solution
|