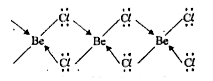
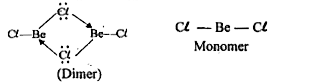Text Solution
Verified by Experts
Topper's Solved these Questions
THE S-BLOCK ELEMENTS
OMEGA PUBLICATION|Exercise Anamalous behaviour of beryllium|2 VideosTHE S-BLOCK ELEMENTS
OMEGA PUBLICATION|Exercise Some important compound of calcium|17 VideosTHE S-BLOCK ELEMENTS
OMEGA PUBLICATION|Exercise Group 2 elements: Alkaline earth metals|11 VideosTHE P-BLOCK ELEMENTS
OMEGA PUBLICATION|Exercise MULTIPLE CHOICE QUESTIONS (MCQs)|28 VideosTHERMODYNAMICS
OMEGA PUBLICATION|Exercise MULTIPLE CHOICE QUESTIONS (MCQ)|25 Videos
Similar Questions
Explore conceptually related problems