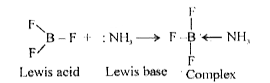Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-THE P-BLOCK ELEMENTS -MULTIPLE CHOICE QUESTIONS (MCQs)
- BF3 acts as a Lewis acid. Why ?
Text Solution
|
- Which of the following configuration is characteristic of group 13- el...
Text Solution
|
- Which of the following element has the highest melting point ?
Text Solution
|
- The element which shows least metallic character
Text Solution
|
- Group 13 elements show
Text Solution
|
- The LE, among the group 13 members follows as
Text Solution
|
- The power of halides of.Boron to act as Lewis acids decreases in the o...
Text Solution
|
- Which of the following is most acidic?
Text Solution
|
- Compounds of Boron with hydrogen are known as
Text Solution
|
- The first ionisation energy of silicon is lower than that of
Text Solution
|
- The stability of +2 oxidation state of Pb can be explained on the basi...
Text Solution
|
- Name the three products each provided by plants and animals?
Text Solution
|
- Halide that is not hydrolysed
Text Solution
|
- name three edible parts of plant?
Text Solution
|
- What is food?
Text Solution
|
- Define the term- Herbivores?
Text Solution
|
- What are carnivores?
Text Solution
|
- What are omnivores?
Text Solution
|
- Make a flow chart of preparation of honey?
Text Solution
|
- ATP molecule is:
Text Solution
|
- Explain the preparation of ghee with the help of flow chart?
Text Solution
|