Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
OMEGA PUBLICATION-HYDROCARBONS-MULTIPLE CHOICE QUESTIONS (MCQs)
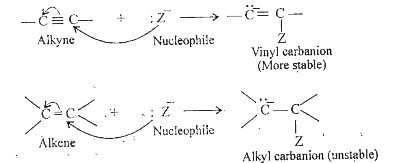
- Why alkynes undergo nucleophilic addition reaction while simple alkene...
Text Solution
|
- Both methane and ethane can be prepared in single step by the use of
Text Solution
|
- Alkyl halides react with Mg in dry ether to form
Text Solution
|
- Which of the following metal can be used for carrying out Wurtz-Fittig...
Text Solution
|
- The most stable conformation of n-butane is
Text Solution
|
- Wurtz reaction involves the interaction of alkyl halides in dry ether ...
Text Solution
|
- Successive alkanes differ by
Text Solution
|
- What are the functions of minerals in our body?
Text Solution
|
- The rational number that does not have a reciprocal.
Text Solution
|
- In the Friedel-Crafts synthesis of toluene, reactants in addition to a...
Text Solution
|
- Write any two functions of water in our body?
Text Solution
|
- The compound which produces propane on heating with HI in presence of ...
Text Solution
|
- Point out the compound out of the following which has the lowest boili...
Text Solution
|
- What are deficiency diseases?
Text Solution
|
- Give one example of slow change and fast change?
Text Solution
|
- A doctor has recommended you to maintain a diet plan for your good hea...
Text Solution
|
- Baeyer's reagent is
Text Solution
|
- Alkáline KMnO4 oxidizes acetylene to
Text Solution
|
- Describe any two functions of stem of a plant?
Text Solution
|
- What is reticulate venation and give examples?
Text Solution
|
- The highest boiling point is expected for
Text Solution
|