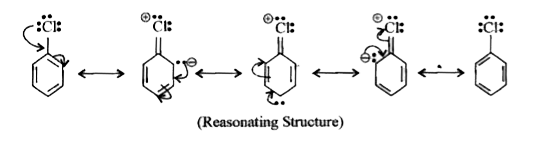
Text Solution
Verified by Experts
Topper's Solved these Questions
HALOALKANES AND HALOARENES
ACCURATE PUBLICATION|Exercise TRUE AND FALSE (1 MARK)|10 VideosHALOALKANES AND HALOARENES
ACCURATE PUBLICATION|Exercise 2 OR 5 MARKS QUESTIONS|23 VideosELECTRO CHEMISTRY
ACCURATE PUBLICATION|Exercise Numbericals Practice|44 VideosIMPORTANT NAMING REACTION
ACCURATE PUBLICATION|Exercise DISTINGUISH TEST|5 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-HALOALKANES AND HALOARENES -4 MARKS QUESTIONS
- Difference between Ape and Man.
Text Solution
|
- Difference between Haloalkene and Haloarenes?
Text Solution
|
- Explain with example S(N^1) mechanism.
Text Solution
|
- Explain SN^2 mechanism ?
Text Solution
|
- Explain the reactivity of SN^2 mechanism ?
Text Solution
|
- Explain the reactivity of SN1 reaction ?
Text Solution
|
- The dipole moment of chlorobenzene is lower than that of cyclohexyl ch...
Text Solution
|
- Why the treatment of alkyl chloride with silver nitrite forms nitroalk...
Text Solution
|
- Reaction of an alkyl halide with KCN and AgCN gives different product...
Text Solution
|
- rhe treatment of alkyl chlorides with aqueous KOH leads to the formati...
Text Solution
|
- Give the following reactions: Fitting reaction
Text Solution
|
- Explain the following reaction: Friedel Craft Acylation
Text Solution
|
- Explain the following reaction: Hunsdicker reaction
Text Solution
|
- Write the following reactions : Friedel Craft alkylation.
Text Solution
|
- Explain the following reactions: Balz Schiemann reaction.
Text Solution
|
- Explain the following : Sulphonation of haloarenes.
Text Solution
|
- Write the following reactions Sandmeyer reaction
Text Solution
|
- Explain the following reaction : Finckelstein reaction
Text Solution
|
- Why alkenes are more reactive than alkanes?
Text Solution
|