Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTRO CHEMISTRY
ACCURATE PUBLICATION|Exercise Numbericals Practice|44 VideosELECTRO CHEMISTRY
ACCURATE PUBLICATION|Exercise 1 Mark Each|22 VideosD & F BLOCK ELEMENTS
ACCURATE PUBLICATION|Exercise 2 OR 5 MARKS QUESTIONS|61 VideosHALOALKANES AND HALOARENES
ACCURATE PUBLICATION|Exercise TRUE AND FALSE (1 MARK)|10 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-ELECTRO CHEMISTRY-3 Marks each
- Define electrochemical cell and electrolytic cell.
Text Solution
|
- Write down the anode, cathode, uses and reactions of dry cell?
Text Solution
|
- Give the cathode, anode, electrolyte and electrode reactions of mercur...
Text Solution
|
- Explain Lead Storage cells?
Text Solution
|
- Explain the working of nickel-cadmium storage cell.
Text Solution
|
- What are fuel cells ? Give example.
Text Solution
|
- What is corrosion?
Text Solution
|
- Explain the effect of more electropositive metal towards the rusting i...
Text Solution
|
- Find the charge in coulombs on 1g ion of N^(-3)
Text Solution
|
- What is mean by galvanization?
Text Solution
|
- Why we can't use AC in electrolyte cells?
Text Solution
|
- Explain the variation in molar conductivity of weak electrolyte with c...
Text Solution
|
- What is the difference between e.m.f. and potential diffrence?
Text Solution
|
- Explain SHE cell?
Text Solution
|
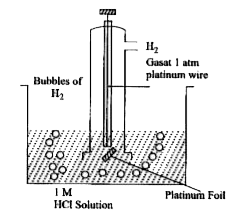
- Describe the construction of standard hydrogen electrode.
Text Solution
|