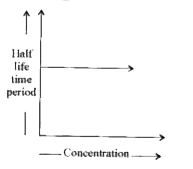
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL KINETICS
ACCURATE PUBLICATION|Exercise NUMERICAL OF HALF LIFE TIME PERIOD |6 VideosCHEMICAL KINETICS
ACCURATE PUBLICATION|Exercise ARRHENIUS EQUATIONS |11 VideosCHEMICAL KINETICS
ACCURATE PUBLICATION|Exercise 1 MARK QUESTIONS|24 VideosBOARD PAPER MARCH - 2020
ACCURATE PUBLICATION|Exercise SECTION - D|18 VideosCOORDINATION COMPOUNDS
ACCURATE PUBLICATION|Exercise 2 MARKS QUESTIONS|23 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-CHEMICAL KINETICS-2 MARK QUESTIONS
- What is the difference between instantaneous rate of a reaction and ra...
Text Solution
|
- Write the difference between molecularity and order of reaction?
Text Solution
|
- Define zero order reaction. Derive integrated rate equation for rate c...
Text Solution
|
- What is the value of integrated Rate reaction in zero order reaction ?
Text Solution
|
- The half life period for a zero order reaction is equal to
Text Solution
|
- Find the half life time period for first order reaction.
Text Solution
|
- Discuss key concept of evolution theory of Darwin.
Text Solution
|
- What are two main points of collision theory?
Text Solution
|
- what is Arrhenius equation .
Text Solution
|