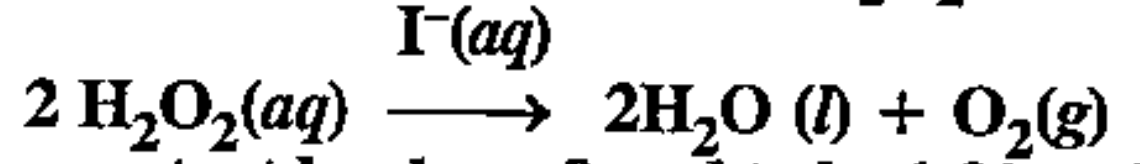Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-CHEMICAL KINETICS-TOPIC : CHEMICAL KINETIC
- State the order with respect to each reactant and overall order for th...
Text Solution
|
- 2NOBr rarr 2NO(g)+Br2(g) Rate=k[NOBr]2 What are the units of ra...
Text Solution
|
- State the order with respect to each reactant and overall order for fo...
Text Solution
|
- Reaction between NO2 and F2 to give NO2F takes place by the following ...
Text Solution
|
- Calculate two third life of first order reaction having K = 5.48 xx 10...
Text Solution
|
- Thermal decomposition of dinitrogen pentoxide takes place by the follo...
Text Solution
|
- The rate constant for the first order reaction is 3.0 xx 10^(-4) "min"...
Text Solution
|
- Thermal decomposition of dinitrogen pentoxide takes place by the follo...
Text Solution
|
- The rate constant for the first order reaction is 60s^(-1). How much t...
Text Solution
|
- A first order reaction is 20% complete in 20 minuts. Calculate the tim...
Text Solution
|
- A first order reaction is 20% complete in the 10 minutes. Calculate th...
Text Solution
|
- Calculate the time required for the completion of 90% of a reaction of...
Text Solution
|
- The decomposition of hydrogen peroxide in the presence of iodide ion h...
Text Solution
|
- The decomposition of N2 O5 in carbon tetrachloride solution has been ...
Text Solution
|
- The decomposition of H2 O2, in the presence of Iodide ion has been fou...
Text Solution
|
- The rate law for a reaction is Rate = k[A]^(1//2)[B]^2 Can the...
Text Solution
|
- Calculate the e.m.f. of the following cell at 298 K, M g ∣ ∣ M g 2 + (...
Text Solution
|
- Calculate the half life period of a first order reaction where specifi...
Text Solution
|
- Find the half life period of first order reaction whose rate constant,...
Text Solution
|
- The rate constant for a first order reaction Is 90 s^-1 .How much time...
Text Solution
|