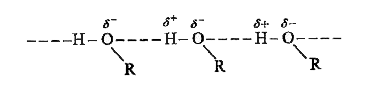Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOL, PHENOL AND ETHER
ACCURATE PUBLICATION|Exercise ETHERS|16 VideosALCOHOL, PHENOL AND ETHER
ACCURATE PUBLICATION|Exercise TRUE AND FALSE (1 MARK) |15 VideosMODEL TEST PAPER-6
ACCURATE PUBLICATION|Exercise SECTION-D (LONG ANSWER QUESTIONS) (TYPE II)|16 VideosALDEHYDES ,KETONES AND CARBOOXALIC ACIDS
ACCURATE PUBLICATION|Exercise DISTINGUISH TEST |5 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-ALCOHOL, PHENOL AND ETHER-3 MARK QUESTIONS
- Discuss the oxidation of alcohols.
Text Solution
|
- Why do alcohols have higher boiling points than halo-alkanes of the sa...
Text Solution
|
- Why are alcohols comparatively more soluble in water than the correspo...
Text Solution
|
- Why alcohols are weaker acids than water ?
Text Solution
|
- Discuss the acidic dehydration of alcohols at different temperatures.
Text Solution
|
- Write the reactions of alcohols with : Sodium
Text Solution
|
- Write the reactions of alcohols with : HI
Text Solution
|
- Write the reactions of alcohols with: HCl
Text Solution
|
- Write the reactions of alcohols with: Sodium hydroxide
Text Solution
|
- Write Victor Meyer's test to distinguish between 1^@, 2^@ and 3^@ alc...
Text Solution
|
- Discuss the reaction and mechanism of acidic dehydration of ethyl alco...
Text Solution
|
- Write two uses of methanol.
Text Solution
|
- Write two uses of ethanol.
Text Solution
|
- Convert the following : 1- Propanol into 2- Propanol.
Text Solution
|
- Why solubility of alcohols in water decreases with increase in molecul...
Text Solution
|
- Why primary alcohols are more acidic than secondary alcohols?
Text Solution
|
- How will you prepare ethanol from : Ethene.
Text Solution
|
- How can you distinguish primary, secondary and teriary alcohols by Luc...
Text Solution
|
- What happens when 1^@, 2^@ and 3^@ alcohols are passed over red hot co...
Text Solution
|
- What are phenols ?
Text Solution
|