Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOL, PHENOL AND ETHER
ACCURATE PUBLICATION|Exercise ETHERS|16 VideosALCOHOL, PHENOL AND ETHER
ACCURATE PUBLICATION|Exercise TRUE AND FALSE (1 MARK) |15 VideosMODEL TEST PAPER-6
ACCURATE PUBLICATION|Exercise SECTION-D (LONG ANSWER QUESTIONS) (TYPE II)|16 VideosALDEHYDES ,KETONES AND CARBOOXALIC ACIDS
ACCURATE PUBLICATION|Exercise DISTINGUISH TEST |5 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-ALCOHOL, PHENOL AND ETHER-3 MARK QUESTIONS
- What are phenols ?
Text Solution
|
- Write Schotten Baumann reaction.
Text Solution
|
- Explain Reimer Tiemann reaction with one example.
Text Solution
|
- Explain Dow 's process .
Text Solution
|
- What is Kolbe’s reaction to prepare salicylic acid ?
Text Solution
|
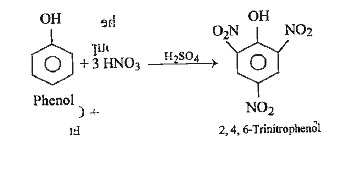
- How is phenol converted into picric acid ?
Text Solution
|
- Alcohols are easily protonated in comparison to phenols.
Text Solution
|
- Orthonitrophenol and paranitrophenol are more acidic than phenols. Giv...
Text Solution
|
- Why phenols are acidic in nature ?
Text Solution
|
- Write short notes on Coupling reaction
Text Solution
|
- How will you convert Phenol to Benzoic acid ?
Text Solution
|
- Why has phenol, higher boiling point than toluene ?
Text Solution
|
- How Phenol is converted to Benzene ?
Text Solution
|
- How does phenol react with Zinc dust.
Text Solution
|
- Write a test to distinguish alcohols from phenols.
Text Solution
|
- Give the chemical reactions for the preparation of phenol from cumene.
Text Solution
|
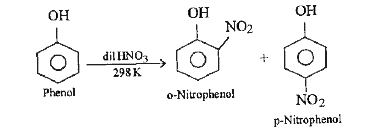
- How does the nitration of phenol with dilute nitric acid differ from n...
Text Solution
|
- How will you prepare phenol from (a) Grignard reagent (b) Benzene
Text Solution
|
- How will you prepare phenol from (a) Grignard reagent (b) Benzene
Text Solution
|
- Write short note on Hoffmann's bromamide reaction. Why is it regarded ...
Text Solution
|