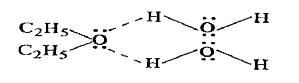
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-ALCOHOL, PHENOL AND ETHER-ETHERS
- What are ethers ?
Text Solution
|
- How diethyl ether reacts with HI
Text Solution
|
- Write two uses of ethers.
Text Solution
|
- Why are ethers relatively inert compounds ?
Text Solution
|
- Why di-tertiary butyl ether cannot be prepared byWilliamson’s synthesi...
Text Solution
|
- The Boiling Point of ethers are lower than isomeric alcohols why ?
Text Solution
|
- Ethers possess a dipole moment even if the alkyl groups in the molecul...
Text Solution
|
- How do you account for the miscibility of ethoxy ethane with water.
Text Solution
|
- Give the functional isomer of CH3CH2OH.
Text Solution
|
- Explain Williamson’ssynthesis.
Text Solution
|
- The Boiling Point of ethers are lower than isomeric alcohols why ?
Text Solution
|
- C-O-C bond angle in ethers is higher than H-O-H bond angle in water th...
Text Solution
|
- Dimethyl ether is completely soluble in water but diethyl ether is sol...
Text Solution
|
- How diethyl ether reacts with PCl(5)
Text Solution
|
- How diethyl ether reacts with Cl(2)
Text Solution
|
- Phenyl methyl ether reacts with HI to give phenol and methyl iodide an...
Text Solution
|