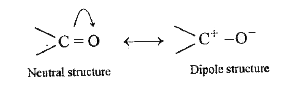
Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES ,KETONES AND CARBOOXALIC ACIDS
ACCURATE PUBLICATION|Exercise DISTINGUISH TEST |5 VideosALDEHYDES ,KETONES AND CARBOOXALIC ACIDS
ACCURATE PUBLICATION|Exercise CARBOXYLIC ACID (1 MARK QUESTIONS )|20 VideosALCOHOL, PHENOL AND ETHER
ACCURATE PUBLICATION|Exercise ETHERS|16 VideosBIOMOLECULES
ACCURATE PUBLICATION|Exercise 2 MARK QUESTIONS |29 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-ALDEHYDES ,KETONES AND CARBOOXALIC ACIDS-CARBOXYLIC ACID (3 MARK QUESTIONS )
- What are strong acid?
Text Solution
|
- Explain Clemmensen's reduction.
Text Solution
|
- Aldehydes have lower boiling points than the corresponding alcohols. E...
Text Solution
|
- Arrange the following compounds in the increasing order of their boili...
Text Solution
|
- Write a chemical test to distinguish between benzaldehyde and acetalde...
Text Solution
|
- Write the chemical equations when acetaldehyde reacts with hydroxyl am...
Text Solution
|
- Write the reaction between acetone and semicarbazide.
Text Solution
|
- Give a chemical test to distinguish between aldehyde and ketone.
Text Solution
|
- What happens when Formaldehyde is treated with ammonia ?
Text Solution
|
- What is formalin solution ? Give its one use..
Text Solution
|
- Two uses of formaldehyde.
Text Solution
|
- What type of aldehyde and ketones undergo Cannizzaro's reaction ?
Text Solution
|
- What type of hybridisation is involved for carbon in a carbonyl group?
Text Solution
|
- The symbol of the elements cobalt , aluminium , helium and sodium resp...
Text Solution
|
- Carboxylic acid exists as dimers, explain why ?
Text Solution
|
- Why are the boiling points of carboxylic acids higher than the corresp...
Text Solution
|
- Carboxylic acids do not give the characteristic reactions of carbonyl ...
Text Solution
|
- Benzoic acid is stronger acid than acetic acid. Justify.
Text Solution
|
- Fluoroacetic acid is stronger than chloroacetic acid. Explain why ?
Text Solution
|
- Why carboxylic acids do not give characteristic reactions of -OH group...
Text Solution
|