A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES ,KETONES AND CARBOOXALIC ACIDS
ACCURATE PUBLICATION|Exercise DISTINGUISH TEST |5 VideosALDEHYDES ,KETONES AND CARBOOXALIC ACIDS
ACCURATE PUBLICATION|Exercise CARBOXYLIC ACID (1 MARK QUESTIONS )|20 VideosALCOHOL, PHENOL AND ETHER
ACCURATE PUBLICATION|Exercise ETHERS|16 VideosBIOMOLECULES
ACCURATE PUBLICATION|Exercise 2 MARK QUESTIONS |29 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-ALDEHYDES ,KETONES AND CARBOOXALIC ACIDS-CARBOXYLIC ACID (3 MARK QUESTIONS )
- What type of aldehyde and ketones undergo Cannizzaro's reaction ?
Text Solution
|
- What type of hybridisation is involved for carbon in a carbonyl group?
Text Solution
|
- The symbol of the elements cobalt , aluminium , helium and sodium resp...
Text Solution
|
- Carboxylic acid exists as dimers, explain why ?
Text Solution
|
- Why are the boiling points of carboxylic acids higher than the corresp...
Text Solution
|
- Carboxylic acids do not give the characteristic reactions of carbonyl ...
Text Solution
|
- Benzoic acid is stronger acid than acetic acid. Justify.
Text Solution
|
- Fluoroacetic acid is stronger than chloroacetic acid. Explain why ?
Text Solution
|
- Why carboxylic acids do not give characteristic reactions of -OH group...
Text Solution
|
- How will you account for the acidic nature of carboxylic acid ?
Text Solution
|
- Benzoic acid is stronger acid than acetic acid. Justify.
Text Solution
|
- How will you account for the acidic nature of carboxylic acid ?
Text Solution
|
- Why acetic acid is weaker than formic acid ?
Text Solution
|
- Why chloroacetic acid is stronger acid than acetic acid ?
Text Solution
|
- Benzoic acid is stronger acid than acetic acid. Justify.
Text Solution
|
- Why trichloro acetic acid is stronger than acetic acid ?
Text Solution
|
- Chloroacetic acid has lower pK(alpha) value than acetic acid. Explain,
Text Solution
|
- Why dichloroacetic acid is stronger than monochloroacetic acid ?
Text Solution
|
- Carboxylic acid exists as dimers, explain why ?
Text Solution
|
- Most aromatic acids are solids while acetic acid and others of their s...
Text Solution
|
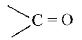 group and alkenes contain
group and alkenes contain 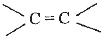 group. (i) On reduction Carbonyl compounds first give alcohols white alkenes give alkanes. (ii) Halogens, halogen acids and H2SO4 undergo addition reactions with alkenes but not with carbonyl compounds.
group. (i) On reduction Carbonyl compounds first give alcohols white alkenes give alkanes. (ii) Halogens, halogen acids and H2SO4 undergo addition reactions with alkenes but not with carbonyl compounds.