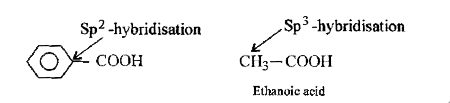Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES ,KETONES AND CARBOOXALIC ACIDS
ACCURATE PUBLICATION|Exercise DISTINGUISH TEST |5 VideosALDEHYDES ,KETONES AND CARBOOXALIC ACIDS
ACCURATE PUBLICATION|Exercise CARBOXYLIC ACID (1 MARK QUESTIONS )|20 VideosALCOHOL, PHENOL AND ETHER
ACCURATE PUBLICATION|Exercise ETHERS|16 VideosBIOMOLECULES
ACCURATE PUBLICATION|Exercise 2 MARK QUESTIONS |29 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-ALDEHYDES ,KETONES AND CARBOOXALIC ACIDS-CARBOXYLIC ACID (3 MARK QUESTIONS )
- Why are the boiling points of carboxylic acids higher than the corresp...
Text Solution
|
- Carboxylic acids do not give the characteristic reactions of carbonyl ...
Text Solution
|
- Benzoic acid is stronger acid than acetic acid. Justify.
Text Solution
|
- Fluoroacetic acid is stronger than chloroacetic acid. Explain why ?
Text Solution
|
- Why carboxylic acids do not give characteristic reactions of -OH group...
Text Solution
|
- How will you account for the acidic nature of carboxylic acid ?
Text Solution
|
- Benzoic acid is stronger acid than acetic acid. Justify.
Text Solution
|
- How will you account for the acidic nature of carboxylic acid ?
Text Solution
|
- Why acetic acid is weaker than formic acid ?
Text Solution
|
- Why chloroacetic acid is stronger acid than acetic acid ?
Text Solution
|
- Benzoic acid is stronger acid than acetic acid. Justify.
Text Solution
|
- Why trichloro acetic acid is stronger than acetic acid ?
Text Solution
|
- Chloroacetic acid has lower pK(alpha) value than acetic acid. Explain,
Text Solution
|
- Why dichloroacetic acid is stronger than monochloroacetic acid ?
Text Solution
|
- Carboxylic acid exists as dimers, explain why ?
Text Solution
|
- Most aromatic acids are solids while acetic acid and others of their s...
Text Solution
|
- Formic acid is stronger acid than acetic acid. Justify.
Text Solution
|
- Benzoic acid is stronger acid than acetic acid. Justify.
Text Solution
|
- p-Nitrobenzoic acid is a stronger acid than benzoic acid. Justify.
Text Solution
|
- Aldehyde are more reactive than ketone ?Explain why.
Text Solution
|