Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN COMPOUNDS
ACCURATE PUBLICATION|Exercise 1 MARK QUESTIONS|22 VideosMODEL TEST PAPER-9
ACCURATE PUBLICATION|Exercise SECTION-D (LONG ANSWER QUESTIONS) (TYPE II)|13 VideosP-BLOCK ELEMENTS
ACCURATE PUBLICATION|Exercise P-BLOCK ELEMENTS GROUP-18 (NOBLE GASES )(2 OR 5 MARK QUESTIONS)|17 Videos
Similar Questions
Explore conceptually related problems
ACCURATE PUBLICATION-ORGANIC COMPOUNDS CONTAINING NITROGEN COMPOUNDS-2 OR 4 MARKS QUESTIONS
- Why is it difficult topreparepure amines by Hofmann’s ammonolysis?
Text Solution
|
- Why is aniline less basic than ethylamine ?
Text Solution
|
- Which is more basic, aliphatic amines or ammonia and why ?
Text Solution
|
- Why lower amines are soluble in water ?
Text Solution
|
- What are tertiary amines ? Give one example
Text Solution
|
- True/False- The mixture of husk and grain can be separated by the proc...
Text Solution
|
- What are primary amines ? Give one example.
Text Solution
|
- Write short note on Gabriel phthalimide synthesis. Why is it regarded ...
Text Solution
|
- Why do primary amines have higher boiling point than tertiary amines?
Text Solution
|
- Out of ammonia (NH(3)) and C(2)H(5)NH(2) which is more basic and why?
Text Solution
|
- Write short note on carbylamine reaction.
Text Solution
|
- Write Hinsberg's test to distinguish between 1^(@), 2^(@) and 3^(@) am...
Text Solution
|
- Why methyl amine has lower boiling point than methanol.
Text Solution
|
- Explain why secondary amines are more basic than primary amines.
Text Solution
|
- Aniline dissolve in aqueous HCl. Why?
Text Solution
|
- How will you convert Aniline to benzene-diazonium chloride ?
Text Solution
|
- How will you convert benzene diazonium chloride to bromobenzene?
Text Solution
|
- Fill in the blanks- is used to separate two immiscible liquids.
Text Solution
|
- How will you convert aniline to benzene ?
Text Solution
|
- How will you convert benzene diazonium chloride to cynobenzene benzoni...
Text Solution
|
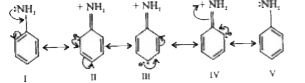 Due to resonance, the lone pair of electron on the nitrogen atom gets delocalised over the benzene ring and thus it is less easily available for protonation. At the same time nitrogen acquires some positive charge (II to IV) and this repel the incoming proton.
Due to resonance, the lone pair of electron on the nitrogen atom gets delocalised over the benzene ring and thus it is less easily available for protonation. At the same time nitrogen acquires some positive charge (II to IV) and this repel the incoming proton.